What is Electron Withdrawing Groups ? | Inductive effect & Acidity | Basics | One Chemistry - YouTube

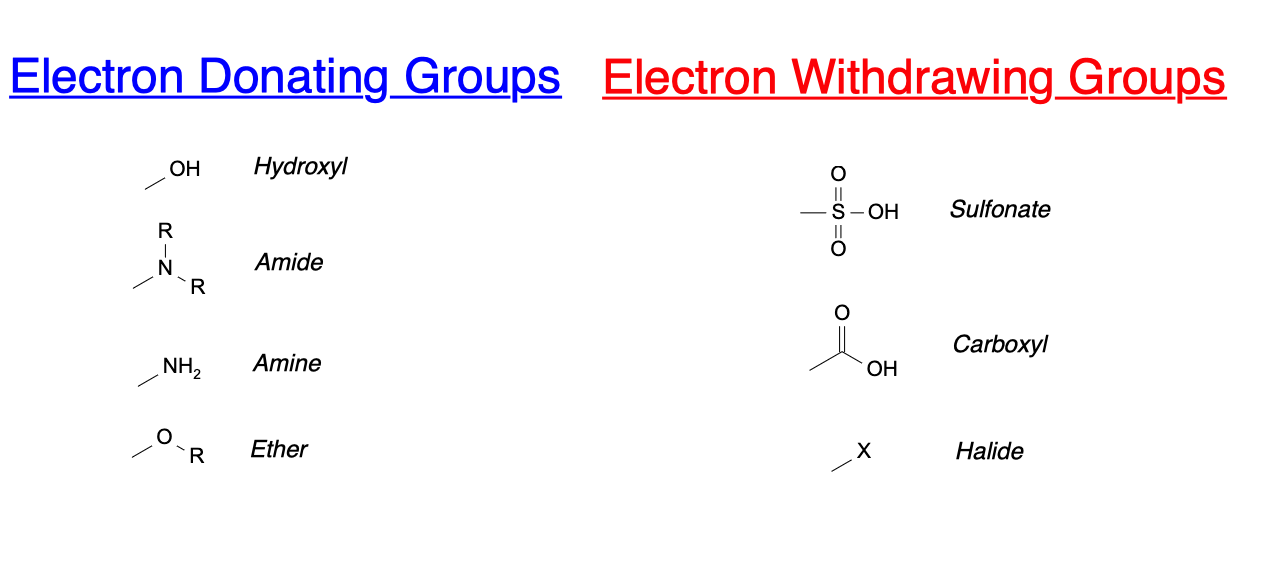
organic chemistry - Amine group - electron donating or withdrawing group? - Chemistry Stack Exchange

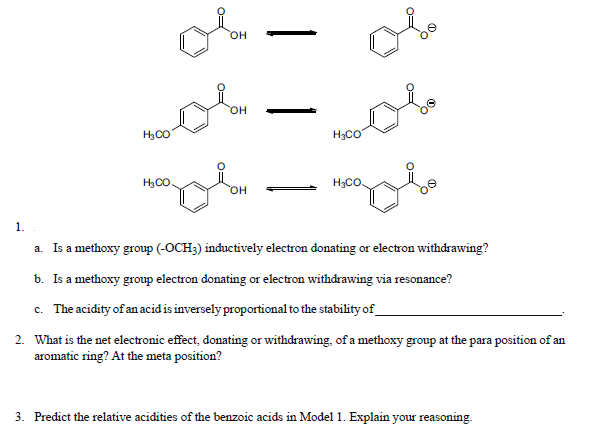
AAMC Question help: Wouldn't the ammonium group also contribute an inductive effect making the R-COOH on the right side acidic as well? If Ammonium is a stronger electron-withdrawing group than chlorine, then

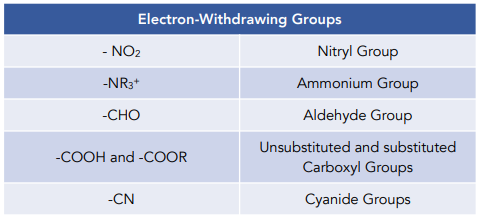
Table 1 from Enhancing effects of electron-withdrawing groups and metallic ions on halogen bonding in the YC6F4X···C2H8N2 (X = Cl, Br, I; Y = F, CN, NO2, LiNC+, NaNC+) complex. | Semantic Scholar
Is it true that fluorine is always the strongest electron withdrawing group (EWG) due to fluorine's unrivaled electronegativity? - Quora

Like alkyl groups are electron releasing groups, are aryl groups electron releasing or electron withdrawing group? - Quora

Dehydrogenative Anodic C−C Coupling of Phenols Bearing Electron‐Withdrawing Groups - Röckl - 2020 - Angewandte Chemie International Edition - Wiley Online Library
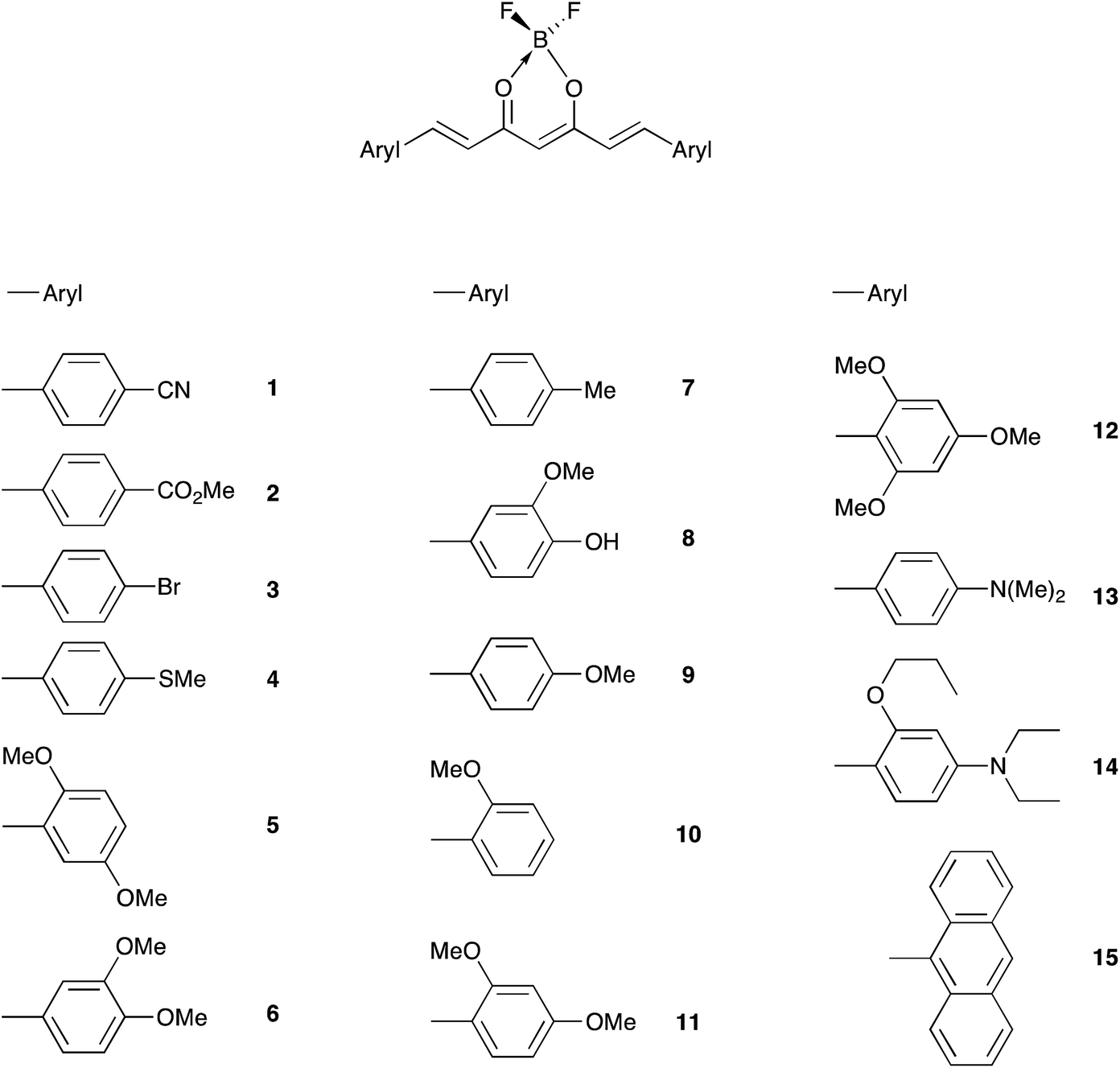
Influence of the electron donor groups on the optical and electrochemical properties of borondifluoride complexes of curcuminoid derivatives: a joint ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA25436E
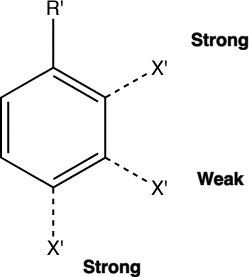
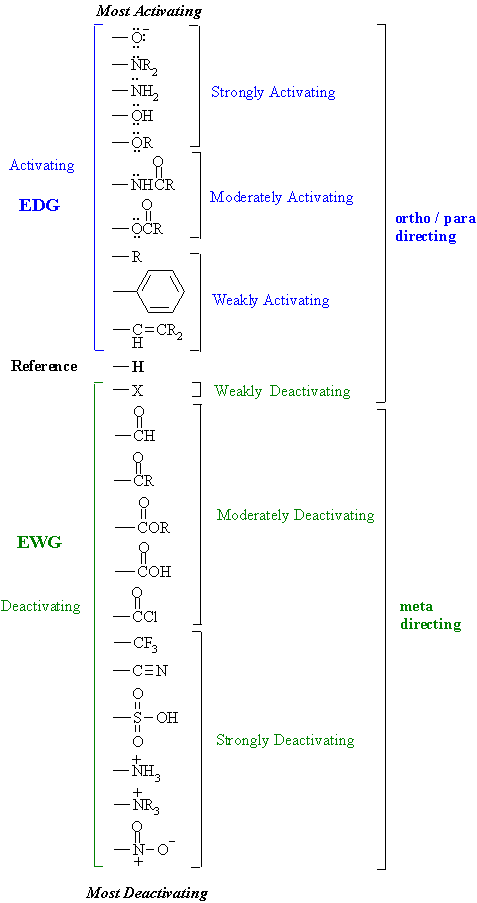
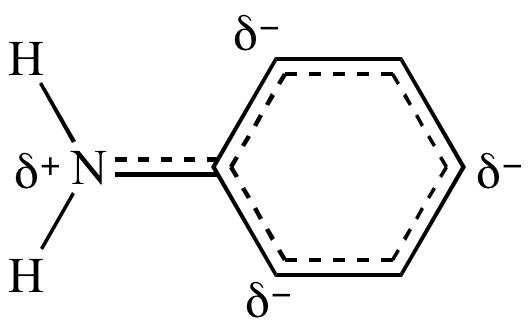
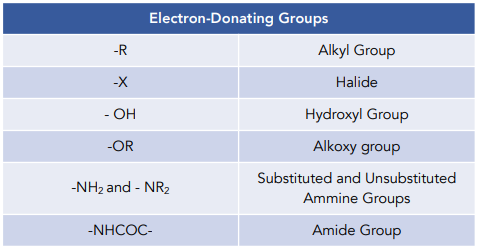
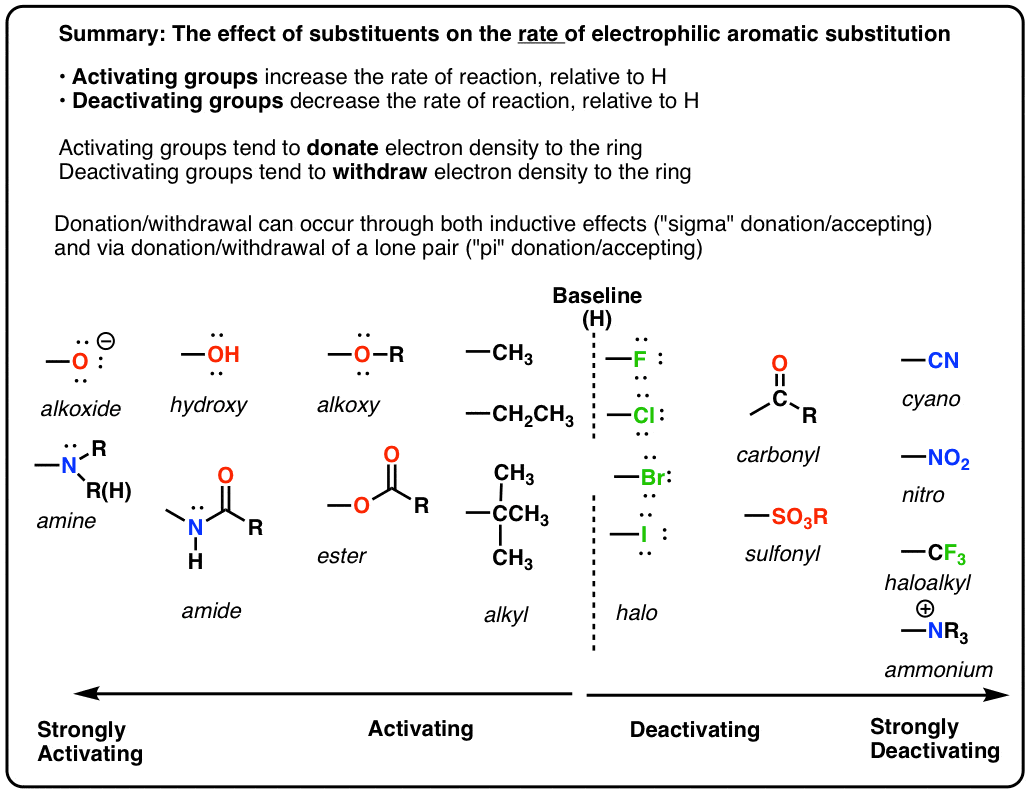
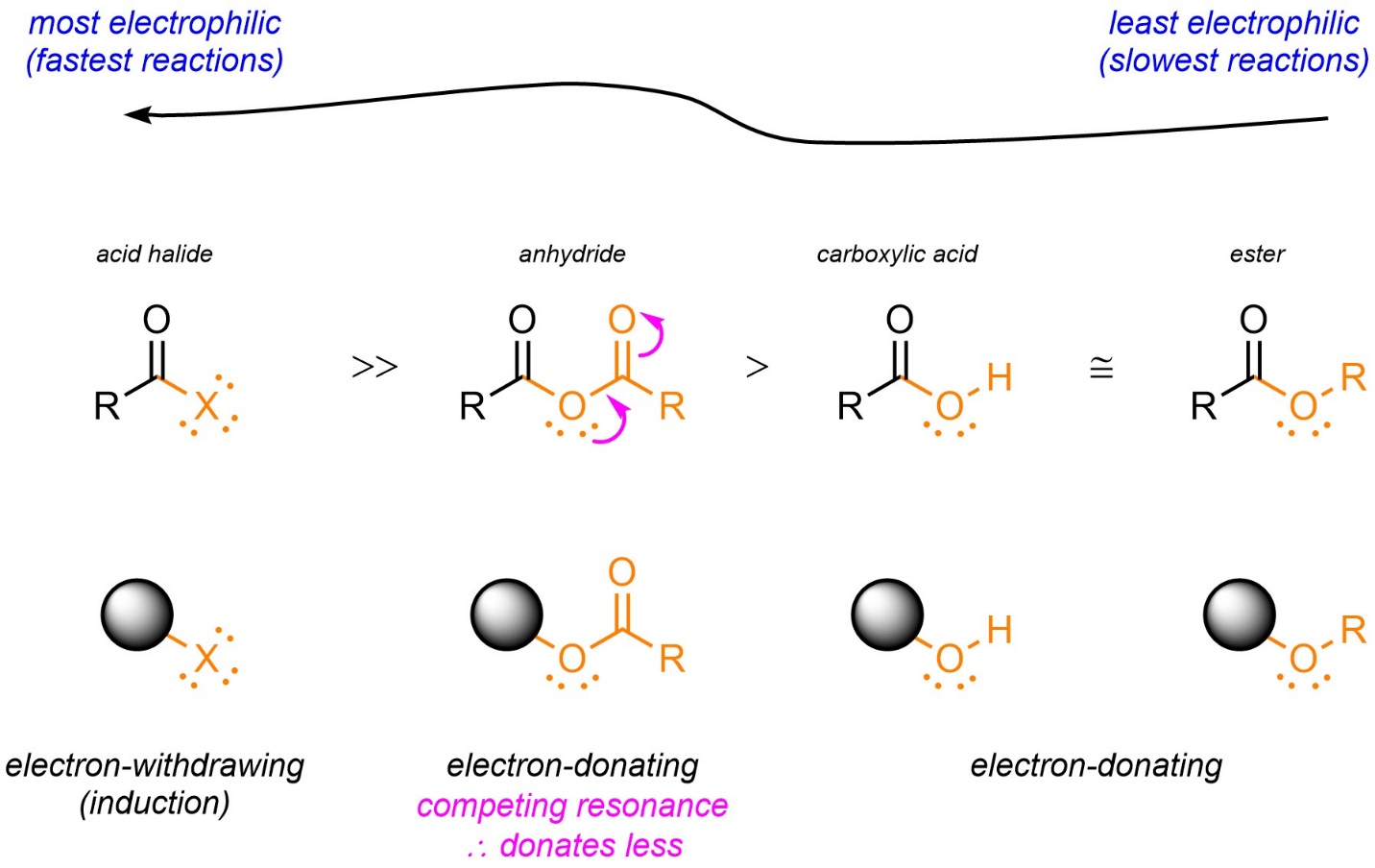
The directing effects of electron donating and withdrawing groups on electrophilic substitution of benzene

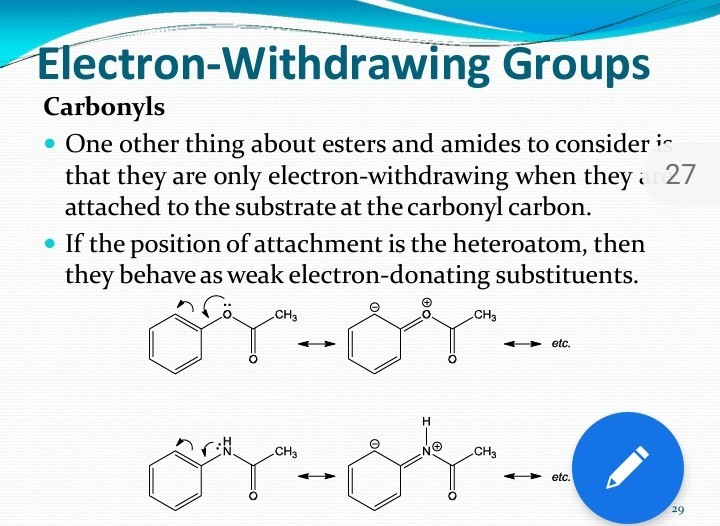
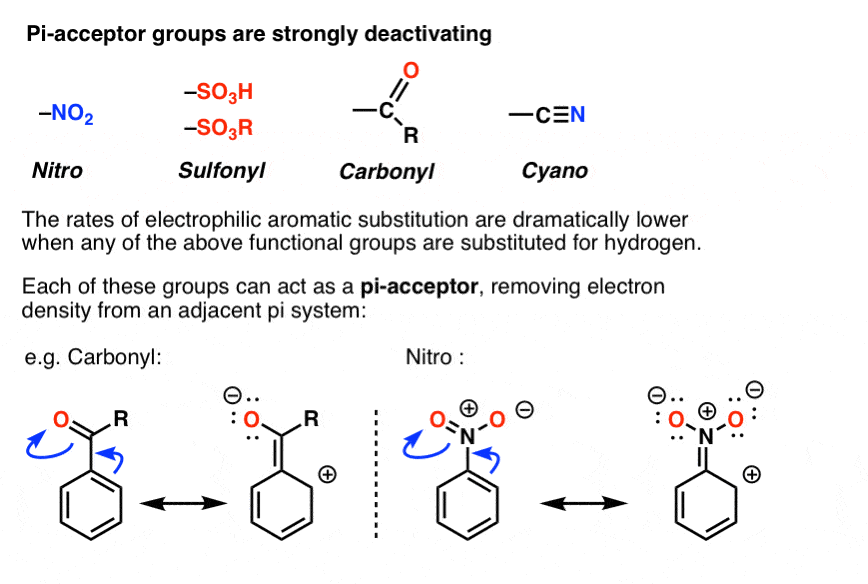
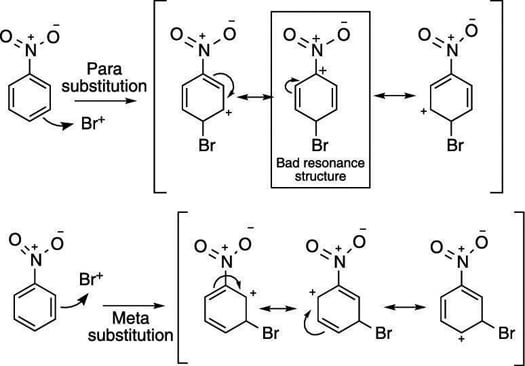
Illustrate using resonance structures how a moderate, to strong, electron-withdrawing group (e.g. -NO2, -COR, etc.) decreases the nucleophilicty of particular sites on an aromatic ring (before the addition of an electrophile).